Fundamental of Electricity
Objectives
- Define matter, element, and molecule.
- List the parts of an atom.
- Define the valence shell of an atom.
- Identify the unit for measuring current.
- Draw the symbol used to represent current in a circuit.
- Describe the difference between conductors and insulators and semiconductors.
- Define difference of potential, electromotive force, and voltage.
- Draw the symbol used to represent voltage.
- Identify the unit used to measure voltage.
- Define resistance.
- Identify characteristics of resistance i na circuit.
- Identify the unit for measuring resistance.
- Draw the symbol used to represent resistance in a circuit.
Matter
Matter is anything that occupies space and has weight.
- Matter is made up of atoms and particles like protons, neutrons, and electrons.
- It exists in different states:
- solid,
- liquid
- gas
- plasma
- more exotic states (like Bose-Einstein condensates)
Atom
- The basic building block of matter. (Each element is made up of only one kind of atom)
- A single atom is defined by the number of protons in its nucleus.
- The number of protons determines the type of atom.
- Example: An atom with 6 protons is a carbon atom.
- Example: An atom with 8 protons is an oxygen atom.
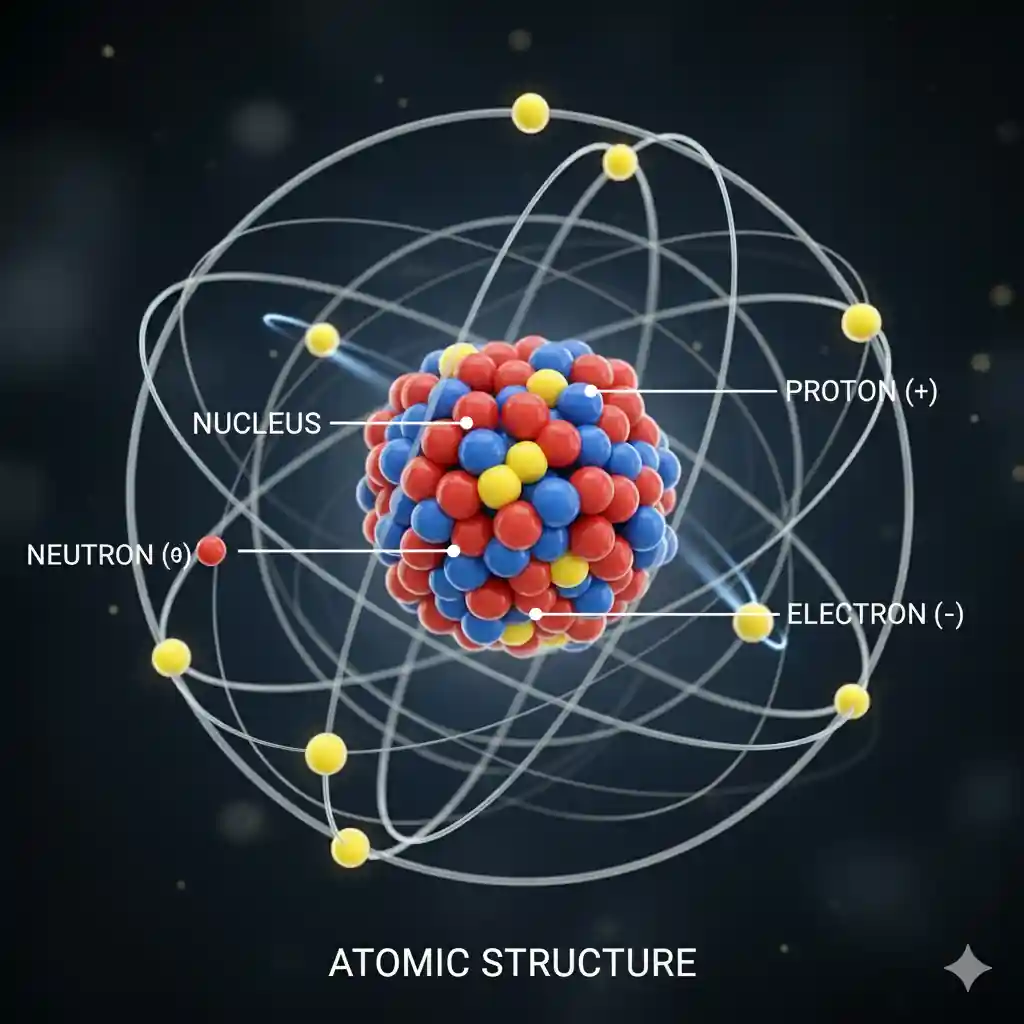
Structure of an Atom
An atom has three main subatomic particles:
- Protons ()
- Positively charged particles
- Found in the nucleus (center of the atom - an area)
- Determines the element (e.g., Hydrogen has 1 proton, Carbon has 6)
- Neutrons ()
- Uncharged particles
- Found in the nucleus
- Adds mass and stabilizes the nucleus
- Electrons ()
- ⚡Negatively charged particles
- 💫Orbit the nucleus in the electron shells or energy levels
- Responsible for chemical reactions and bonding
Key Properties
- 🔢Atomic number (Z): Number of protons in the nucleus. It determines the element.
- ⚖️Atomic weight (Mass number) (A): Total protons + neutrons. The mass of an atom. (just count the number)
- Isotopes: Atoms of the same element with different numbers of neutrons. (???)
- 6 is the atomic number which is also the number of protons in the nucleus.
- 12 is the mass number which is also the number of protons + neutrons.
Calculation:
- Number of Protons (A, atomic number): 6
- Number of Neutrons: Mass - Atomic Number = 12 - 6 = 6
- Number of Electrons: Atomic Number - charge = 6 - 0 = 6
Calculation:
- Number of Protons: 6
- Number of Neturons: 13 - 6 = 7
- Number of Electrons: 6 (it is an atom since it is not charged)
Electron Arrangement
Electrons occupy electron shells around the nucleus. Shells are filled in sequence.
- 1st shell: up to 2 electrons
- 2nd shell: up to 8 electrons
- 3rd shell: up to 18 electrons
This arrangement explains chemical properties and how atoms bound.
- Valence Shell: The outermost shell.
- Valence: The number of electrons contained in the valence shell.
Element
An element is a pure substance made of only one type of atom.
- Each element is made up of ONLY ONE kind of atom.
- Defined by the atomic number (number of protons)
- Example:
- Carbon (C) all atoms have 6 protons.
- Oxygen (O) all atoms have 8 protons.
- Gold (Au) all atoms have 79 protons.
Element could have one atom or trillions of atoms, but all of them are the same type.
Example 1: Gold jewelry 🪙
- One gold atom a single particle with 79 protons.
- The element gold your entire gold ring/necklace is made of trillions of gold atoms, all the same type.
Example 2: Diamond 💎
- One carbon atom a single particle with 6 protons.
- The element carbon a diamond is a pure carbon, but built from billions of carbon atoms linked together in a crystal structure.
Molecule
- A group of two or more atoms bounded together.
- The atoms may be of the same element or different elements.
- Examples:
- a molecule of oxygen (two oxygen atoms, same element).
- a molecule of water (two hydrogen atoms + one oxygen atom, different elements).
Compound
- A compound is a molecule made of atoms of different elements.
- Examples:
- hydrogen + oxygen compound
- carbon + oxygen compound
Every compound is a molecule, but not every molecule is a compound.
Conductors
Materials that contain a large number of free electrons.
If an element has 3 or less electrons in the valence shell, it might be a good conductor.
| Material 🧪 | Conductivity (S/m) ⚡ |
|---|---|
| Silver 🥈 | 6.30 × 10⁷ |
| Copper 🟠 | 5.96 × 10⁷ |
| Gold 🥇 | 4.10 × 10⁷ |
| Aluminum ⚪ | 3.77 × 10⁷ |
| Tungsten ⚫ | 1.79 × 10⁷ |
| Iron 🛠️ | 1.00 × 10⁷ |
| Lead ⚙️ | 4.55 × 10⁶ |
| Carbon (Graphite) 🖤 | 1.00 × 10⁵ |
| Silicon (doped) 💻 | 1.00 × 10⁴ |
| Glass 🔮 | 10⁻¹² – 10⁻¹⁰ |
| Rubber 🟢 | 10⁻¹⁴ – 10⁻¹² |
Insulators
Prevent the flow of electricity. Stabilized by absorbing valence electrons.
5 or more electrons in the valence shell.
Semiconductors
Can be altered to function as either as a conductor or insulator. Made out of materials with 4 electrons in the valence shell.
- Negative Ion (Anion): A negatively charged atom. (e.g., )
- Positive Ion (Cation): A positively charged atom. (e.g., )
Atom has equal number of protons and electrons. Electrical netural
Atom:
- protons: 13
- neturons: 27 - 13 = 14
- electrons: 13
Ion has different numbers of protons and electrons. Charged
Ion:
- protons: 13
- neturons: 27 - 13 = 14
- electrons: atomic number - charge = 13 - (3) = 10
Current
Movement of electrons from negatively charged atoms to positively charged atoms. Represented as .
Coulomb
- electrons.
- Represented as .
Ampere
- One coulomb moving past a single point in one second (). Represented by .
Potential
The ability of the source to perform electrical work.
Difference of potential causes electrons to move or flow in a circuit.
Voltage
The force that moves the electrons in the circuit. Represented by . Unit of measure is volt.
Resistance
- Opposition to the flow of electrons. (reduce current)
- Measured in Homs.
- Represented by
References
- Basic Electronics Part 1 https://www.youtube.com/watch?v=nb4ovfwqup8